Writing your own trajectory analysis
We create our own analysis methods for calculating the radius of gyration of a selection of atoms.
This can be done three ways, from least to most flexible:
The building blocks and methods shown here are only suitable for analyses that involve iterating over the trajectory once.
If you implement your own analysis method, please consider contributing it to the MDAnalysis codebase!
Last updated: December 2022 with MDAnalysis 2.4.0-dev0
Minimum version of MDAnalysis: 2.0.0
Packages required:
[1]:
import MDAnalysis as mda
from MDAnalysis.tests.datafiles import PSF, DCD, DCD2
from MDAnalysis.analysis.base import (AnalysisBase,
AnalysisFromFunction,
analysis_class)
import numpy as np
import pandas as pd
import matplotlib.pyplot as plt
%matplotlib inline
Radius of gyration
Let’s start off by defining a standalone analysis function.
The radius of gyration of a structure measures how compact it is. In GROMACS, it is calculated as follows:
where \(m_i\) is the mass of atom \(i\) and \(\mathbf{r}_i\) is the position of atom \(i\), relative to the center-of-mass of the selection.
The radius of gyration around each axis can also be determined separately. For example, the radius of gyration around the x-axis:
Below, we define a function that takes an AtomGroup and calculates the radii of gyration. We could write this function to only need the AtomGroup. However, we also add in a masses argument and a total_mass keyword to avoid recomputing the mass and total mass for each frame.
[2]:
def radgyr(atomgroup, masses, total_mass=None):
# coordinates change for each frame
coordinates = atomgroup.positions
center_of_mass = atomgroup.center_of_mass()
# get squared distance from center
ri_sq = (coordinates-center_of_mass)**2
# sum the unweighted positions
sq = np.sum(ri_sq, axis=1)
sq_x = np.sum(ri_sq[:,[1,2]], axis=1) # sum over y and z
sq_y = np.sum(ri_sq[:,[0,2]], axis=1) # sum over x and z
sq_z = np.sum(ri_sq[:,[0,1]], axis=1) # sum over x and y
# make into array
sq_rs = np.array([sq, sq_x, sq_y, sq_z])
# weight positions
rog_sq = np.sum(masses*sq_rs, axis=1)/total_mass
# square root and return
return np.sqrt(rog_sq)
Loading files
The test files we will be working with here feature adenylate kinase (AdK), a phosophotransferase enzyme. ([BDPW09])
[3]:
u = mda.Universe(PSF, DCD)
protein = u.select_atoms('protein')
u2 = mda.Universe(PSF, DCD2)
/home/pbarletta/mambaforge/envs/guide/lib/python3.9/site-packages/MDAnalysis/coordinates/DCD.py:165: DeprecationWarning: DCDReader currently makes independent timesteps by copying self.ts while other readers update self.ts inplace. This behavior will be changed in 3.0 to be the same as other readers. Read more at https://github.com/MDAnalysis/mdanalysis/issues/3889 to learn if this change in behavior might affect you.
warnings.warn("DCDReader currently makes independent timesteps"
Creating an analysis from a function
MDAnalysis.analysis.base.AnalysisFromFunction can create an analysis from a function that works on AtomGroups. It requires the function itself, the trajectory to operate on, and then the arguments / keyword arguments necessary for the function.
[4]:
rog = AnalysisFromFunction(radgyr, u.trajectory,
protein, protein.masses,
total_mass=np.sum(protein.masses))
rog.run()
[4]:
<MDAnalysis.analysis.base.AnalysisFromFunction at 0x7f901a31bca0>
Running the analysis iterates over the trajectory. The output is saved in rog.results.timeseries, which has the same number of rows, as frames in the trajectory. You can access the results both at rog.results.timeseries and rog.results['timeseries']:
[5]:
rog.results['timeseries'].shape
[5]:
(98, 4)
gives the same outputs as:
[6]:
rog.results.timeseries.shape
[6]:
(98, 4)
[7]:
labels = ['all', 'x-axis', 'y-axis', 'z-axis']
for col, label in zip(rog.results['timeseries'].T, labels):
plt.plot(col, label=label)
plt.legend()
plt.ylabel('Radius of gyration (Å)')
plt.xlabel('Frame')
[7]:
Text(0.5, 0, 'Frame')
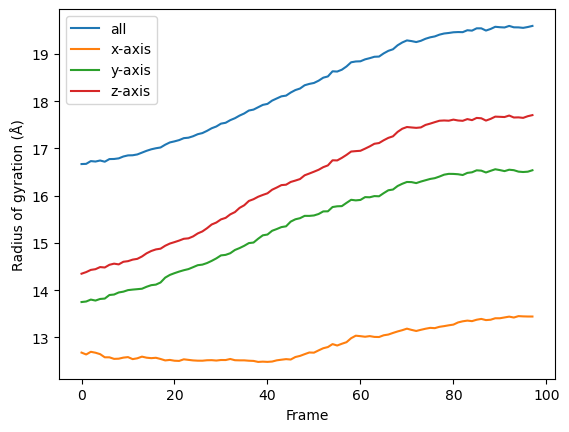
You can also re-run the analysis with different frame selections.
Below, we start from the 10th frame and take every 8th frame until the 80th. Note that the slice includes the start frame, but does not include the stop frame index (much like the actual range() function).
[8]:
rog_10 = AnalysisFromFunction(radgyr, u.trajectory,
protein, protein.masses,
total_mass=np.sum(protein.masses))
rog_10.run(start=10, stop=80, step=7)
rog_10.results['timeseries'].shape
[8]:
(10, 4)
[9]:
for col, label in zip(rog_10.results['timeseries'].T, labels):
plt.plot(col, label=label)
plt.legend()
plt.ylabel('Radius of gyration (Å)')
plt.xlabel('Frame')
[9]:
Text(0.5, 0, 'Frame')
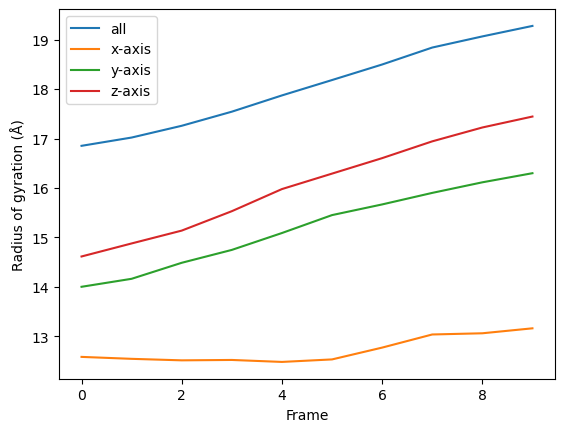
Transforming a function into a class
While the AnalysisFromFunction is convenient for quick analyses, you may want to turn your function into a class that can be applied to many different trajectories, much like other MDAnalysis analyses.
You can apply analysis_class to any function that you can run with AnalysisFromFunction to get a class.
[10]:
RadiusOfGyration = analysis_class(radgyr)
To run the analysis, pass exactly the same arguments as you would for AnalysisFromFunction.
[11]:
rog_u1 = RadiusOfGyration(u.trajectory, protein,
protein.masses,
total_mass=np.sum(protein.masses))
rog_u1.run()
[11]:
<MDAnalysis.analysis.base.analysis_class.<locals>.WrapperClass at 0x7f9000500820>
As with AnalysisFromFunction, the results are in results.
[12]:
for col, label in zip(rog_u1.results['timeseries'].T, labels):
plt.plot(col, label=label)
plt.legend()
plt.ylabel('Radius of gyration (Å)')
plt.xlabel('Frame')
[12]:
Text(0.5, 0, 'Frame')
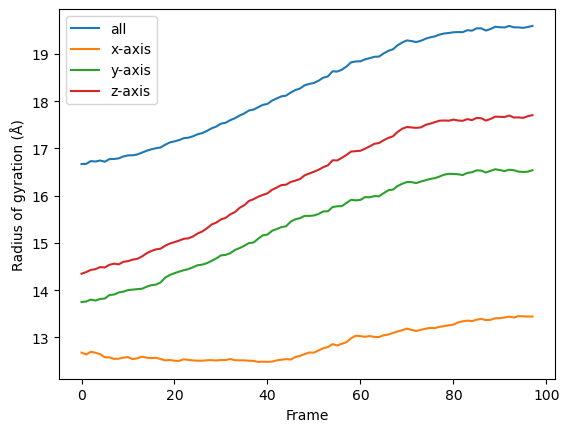
You can reuse the class for other trajectories and selections.
[13]:
ca = u2.select_atoms('name CA')
rog_u2 = RadiusOfGyration(u2.trajectory, ca,
ca.masses,
total_mass=np.sum(ca.masses))
rog_u2.run()
[13]:
<MDAnalysis.analysis.base.analysis_class.<locals>.WrapperClass at 0x7f9000474df0>
[14]:
for col, label in zip(rog_u2.results['timeseries'].T, labels):
plt.plot(col, label=label)
plt.legend()
plt.ylabel('Radius of gyration (Å)')
plt.xlabel('Frame')
[14]:
Text(0.5, 0, 'Frame')
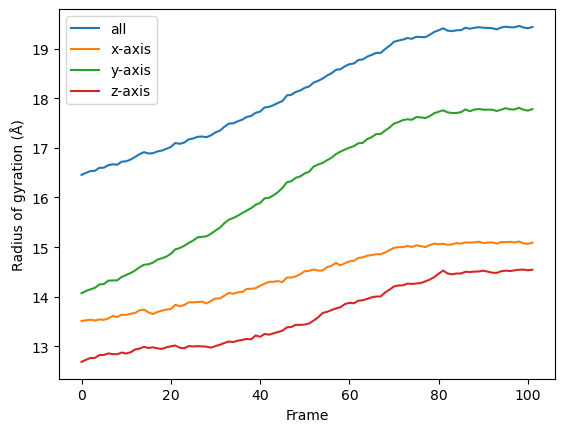
Creating your own class
Although AnalysisFromFunction and analysis_class are convenient, they can be too limited for complex algorithms. You may need to write your own class.
MDAnalysis provides the MDAnalysis.analysis.base.AnalysisBase class as a template for creating multiframe analyses. This class automatically sets up your trajectory reader for iterating, and includes an optional progress meter.
The analysis is always run by calling run(). AnalysisFromFunction actually subclasses AnalysisBase, and analysis_class returns a subclass of AnalysisFromFunction, so the behaviour of run() remains identical.
1. Define __init__
You can define a new analysis by subclassing AnalysisBase. Initialise the analysis with the __init__ method, where you must pass the trajectory that you are working with to AnalysisBase.__init__(). You can also pass in the verbose keyword. If verbose=True, the class will set up a progress meter for you.
2. Define your analysis in _single_frame() and other methods
Implement your functionality as a function over each frame of the trajectory by defining _single_frame(). This function gets called for each frame of your trajectory.
You can also define _prepare() and _conclude() to set your analysis up before looping over the trajectory, and to finalise the results that you have prepared. In order, run() calls:
_prepare()_single_frame()(for each frame of the trajectory that you are iterating over)_conclude()
Class subclassed from AnalysisBase can make use of several properties when defining the methods above:
self.start: frame index to start analysing from. Defined inrun()self.stop: frame index to stop analysis. Defined inrun()self.step: number of frames to skip in between. Defined inrun()self.n_frames: number of frames to analyse over. This can be helpful in initialising result arrays.self._verbose: whether to be verbose.self._trajectory: the actual trajectoryself._ts: the current timestep objectself._frame_index: the index of the currently analysed frame. This is not the absolute index of the frame in the trajectory overall, but rather the relative index of the frame within the list of frames to be analysed. You can think of it as the number of times thatself._single_frame()has already been called.
Below, we create the class RadiusOfGyration2 to run the analysis function that we have defined above, and add extra information such as the time of the corresponding frame.
[15]:
class RadiusOfGyration2(AnalysisBase): # subclass AnalysisBase
def __init__(self, atomgroup, verbose=True):
"""
Set up the initial analysis parameters.
"""
# must first run AnalysisBase.__init__ and pass the trajectory
trajectory = atomgroup.universe.trajectory
super(RadiusOfGyration2, self).__init__(trajectory,
verbose=verbose)
# set atomgroup as a property for access in other methods
self.atomgroup = atomgroup
# we can calculate masses now because they do not depend
# on the trajectory frame.
self.masses = self.atomgroup.masses
self.total_mass = np.sum(self.masses)
def _prepare(self):
"""
Create array of zeroes as a placeholder for results.
This is run before we begin looping over the trajectory.
"""
# This must go here, instead of __init__, because
# it depends on the number of frames specified in run().
self.results = np.zeros((self.n_frames, 6))
# We put in 6 columns: 1 for the frame index,
# 1 for the time, 4 for the radii of gyration
def _single_frame(self):
"""
This function is called for every frame that we choose
in run().
"""
# call our earlier function
rogs = radgyr(self.atomgroup, self.masses,
total_mass=self.total_mass)
# save it into self.results
self.results[self._frame_index, 2:] = rogs
# the current timestep of the trajectory is self._ts
self.results[self._frame_index, 0] = self._ts.frame
# the actual trajectory is at self._trajectory
self.results[self._frame_index, 1] = self._trajectory.time
def _conclude(self):
"""
Finish up by calculating an average and transforming our
results into a DataFrame.
"""
# by now self.result is fully populated
self.average = np.mean(self.results[:, 2:], axis=0)
columns = ['Frame', 'Time (ps)', 'Radius of Gyration',
'Radius of Gyration (x-axis)',
'Radius of Gyration (y-axis)',
'Radius of Gyration (z-axis)',]
self.df = pd.DataFrame(self.results, columns=columns)
Because RadiusOfGyration2 calculates the masses of the selected AtomGroup itself, we do not need to pass it in ourselves.
[16]:
rog_base = RadiusOfGyration2(protein, verbose=True).run()
As calculated in _conclude(), the average radii of gyrations are at rog.average.
[17]:
rog_base.average
[17]:
array([18.26549552, 12.85342131, 15.37359575, 16.29185734])
The results are available at rog.results as an array or rog.df as a DataFrame.
[18]:
rog_base.df
[18]:
| Frame | Time (ps) | Radius of Gyration | Radius of Gyration (x-axis) | Radius of Gyration (y-axis) | Radius of Gyration (z-axis) | |
|---|---|---|---|---|---|---|
| 0 | 0.0 | 1.000000 | 16.669018 | 12.679625 | 13.749343 | 14.349043 |
| 1 | 1.0 | 2.000000 | 16.673217 | 12.640025 | 13.760545 | 14.382960 |
| 2 | 2.0 | 3.000000 | 16.731454 | 12.696454 | 13.801342 | 14.429350 |
| 3 | 3.0 | 4.000000 | 16.722283 | 12.677194 | 13.780732 | 14.444711 |
| 4 | 4.0 | 5.000000 | 16.743961 | 12.646981 | 13.814553 | 14.489046 |
| ... | ... | ... | ... | ... | ... | ... |
| 93 | 93.0 | 93.999992 | 19.562034 | 13.421683 | 16.539112 | 17.653968 |
| 94 | 94.0 | 94.999992 | 19.560575 | 13.451335 | 16.508649 | 17.656678 |
| 95 | 95.0 | 95.999992 | 19.550571 | 13.445914 | 16.500640 | 17.646130 |
| 96 | 96.0 | 96.999991 | 19.568381 | 13.443243 | 16.507396 | 17.681294 |
| 97 | 97.0 | 97.999991 | 19.591575 | 13.442750 | 16.537926 | 17.704494 |
98 rows × 6 columns
Using this DataFrame we can easily plot our results.
[19]:
ax = rog_base.df.plot(x='Time (ps)', y=rog_base.df.columns[2:])
ax.set_ylabel('Radius of gyration (A)')
[19]:
Text(0, 0.5, 'Radius of gyration (A)')
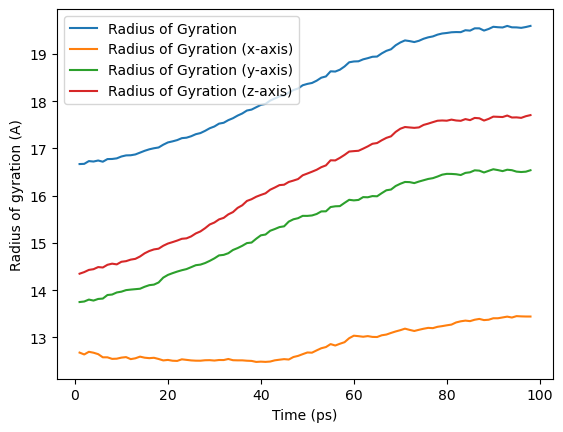
We can also run the analysis over a subset of frames, the same as the output from AnalysisFromFunction and analysis_class.
[20]:
rog_base_10 = RadiusOfGyration2(protein, verbose=True)
rog_base_10.run(start=10, stop=80, step=7)
[20]:
<__main__.RadiusOfGyration2 at 0x7f90006ca9d0>
[21]:
rog_base_10.results.shape
[21]:
(10, 6)
[22]:
rog_base_10.df
[22]:
| Frame | Time (ps) | Radius of Gyration | Radius of Gyration (x-axis) | Radius of Gyration (y-axis) | Radius of Gyration (z-axis) | |
|---|---|---|---|---|---|---|
| 0 | 10.0 | 10.999999 | 16.852127 | 12.584163 | 14.001589 | 14.614469 |
| 1 | 17.0 | 17.999998 | 17.019587 | 12.544784 | 14.163276 | 14.878262 |
| 2 | 24.0 | 24.999998 | 17.257429 | 12.514341 | 14.487021 | 15.137873 |
| 3 | 31.0 | 31.999997 | 17.542565 | 12.522147 | 14.747461 | 15.530339 |
| 4 | 38.0 | 38.999997 | 17.871241 | 12.482385 | 15.088865 | 15.977444 |
| 5 | 45.0 | 45.999996 | 18.182243 | 12.533023 | 15.451285 | 16.290153 |
| 6 | 52.0 | 52.999995 | 18.496493 | 12.771949 | 15.667003 | 16.603098 |
| 7 | 59.0 | 59.999995 | 18.839346 | 13.037335 | 15.900327 | 16.942533 |
| 8 | 66.0 | 66.999994 | 19.064333 | 13.061491 | 16.114195 | 17.222884 |
| 9 | 73.0 | 73.999993 | 19.276639 | 13.161863 | 16.298539 | 17.444213 |
[23]:
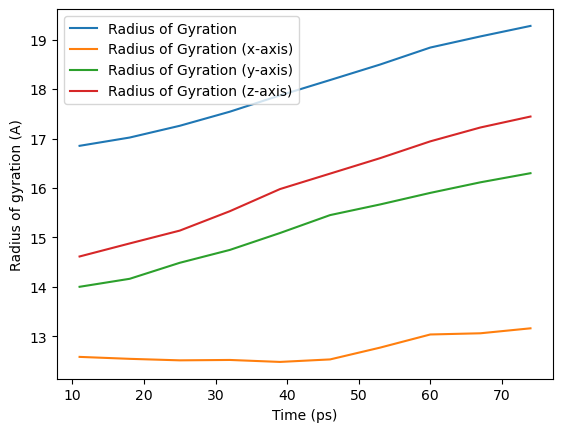
ax_10 = rog_base_10.df.plot(x='Time (ps)',
y=rog_base_10.df.columns[2:])
ax_10.set_ylabel('Radius of gyration (A)')
[23]:
Text(0, 0.5, 'Radius of gyration (A)')
Contributing to MDAnalysis
If you think that you will want to reuse your new analysis, or that others might find it helpful, please consider contributing it to the MDAnalysis codebase. Making your code open-source can have many benefits; others may notice an unexpected bug or suggest ways to optimise your code. If you write your analysis for a specific publication, please let us know; we will ask those who use your code to cite your reference in published work.
References
[1] Oliver Beckstein, Elizabeth J. Denning, Juan R. Perilla, and Thomas B. Woolf. Zipping and Unzipping of Adenylate Kinase: Atomistic Insights into the Ensemble of Open↔Closed Transitions. Journal of Molecular Biology, 394(1):160–176, November 2009.
URL: https://linkinghub.elsevier.com/retrieve/pii/S0022283609011164, doi:10.1016/j.jmb.2009.09.009.
[2] Richard J. Gowers, Max Linke, Jonathan Barnoud, Tyler J. E. Reddy, Manuel N. Melo, Sean L. Seyler, Jan Domański, David L. Dotson, Sébastien Buchoux, Ian M. Kenney, and Oliver Beckstein. MDAnalysis: A Python Package for the Rapid Analysis of Molecular Dynamics Simulations. Proceedings of the 15th Python in Science Conference, pages 98–105, 2016.
URL: https://conference.scipy.org/proceedings/scipy2016/oliver_beckstein.html, doi:10.25080/Majora-629e541a-00e.
[3] Naveen Michaud-Agrawal, Elizabeth J. Denning, Thomas B. Woolf, and Oliver Beckstein. MDAnalysis: A toolkit for the analysis of molecular dynamics simulations. Journal of Computational Chemistry, 32(10):2319–2327, July 2011.
URL: http://doi.wiley.com/10.1002/jcc.21787, doi:10.1002/jcc.21787.